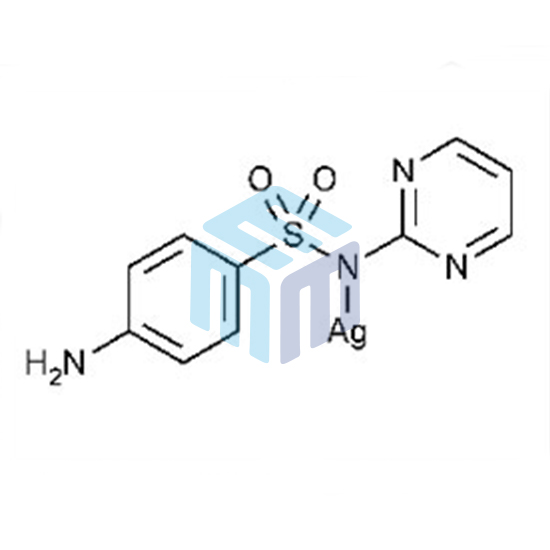
Silver Sulfadiazine USP
Inquiry NowProduct Description
PRODUCT SPECIFICATIONS
| Name of Product: | SILVER SULFADIAZINE USP |
| Synonyms: | (4-Amino-N-2-pyrimidinylbenzenesulfonamidato-NN,01)-silver, sulfadiazine silver, silver (I) sulfadiazine, 4-amino-N-(2-pyrimidinyl) benzenesulfonamide silver salt, dermazine, geben, silvadene |
| CAS No: | 22199-08-2 |
SPECIFICATIONS
| Sr. No | Criteria | Limit/Specification | ||||||||||||||||||
| 1. | Appearance | White to creamy- white, crystalline powder. Odorless to having it slight odor. Is stable in air, but turns yellow on exposure to light. | ||||||||||||||||||
| 2. | Solubility | Freely soluble in 30% of ammonia solution; slightly soluble in acetone; practically insoluble in ethanol (95%), chloroform and ether. | ||||||||||||||||||
| 3. | Identification | |||||||||||||||||||
| A. By IR | The IR Spectrum is concordant with the reference spectrum of Silver Sulfadiazine | |||||||||||||||||||
| B. By HPLC | The RT of the major peak of the Sample solution corresponds t O that of the Standard solution, as obtained in the Assay | |||||||||||||||||||
| C. Test for Silver | Meets the Requirement of test for Silver | |||||||||||||||||||
| 4. | Silver Content | 29.3% – 30.5% of Silver is found | ||||||||||||||||||
| 5. | Limit of Nitrate (By UV-Vis) | Nmt 0.5% | ||||||||||||||||||
| 6. | Organic Impurities (By HPLC ) |
|
||||||||||||||||||
| 7. | Loss of Drying | NMT 0.5% | ||||||||||||||||||
| 8. | Assay (By HPLC) | 98.0% – 102.0% on the dried basis | ||||||||||||||||||
M-Kube Enterprise is an Australian company catering customized laboratory products, laboratory consumables, and laboratory solutions in Australia, Dubai, India, Indonesia, Malaysia, New Zealand, the Philippines, Singapore, South Korea, the USA, and Vietnam. Our team of experts across different platforms can discuss and customize your requirements as per your needs.
Please reach out to us on info@mkube.com.au or call us on +61478594746 to discuss your projects.